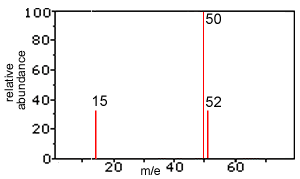
1) Explain the presence of two lines at the m/e values of 50 and 52.Solution
2) Explain the presence of the line at the m/e values of 15. Solution
3) On the basis of this mass spectrum, determine whether the product is chloromethane or dichloromethane? Explain your choice. Solution
4) According to the mass spectrum is the relative molecular mass of chloromethane closer to 50 or 52? Explain. Solution
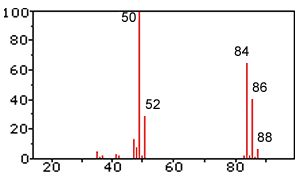
1) Explain the presence of a line at 88 and at 52. Solution
2) What does the line at 50 represent? Solution
3) What does the line at 86 and 84 represent? Solution
4) Judging by this spectrum is the product a pure substance? Explain Solution
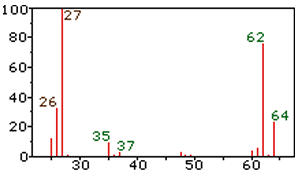
1) What is the likely relative molecular mass of this compound. Solution
2) What is the likely formula of this compound? Solution
3) What does the line at 27 represent? Solution